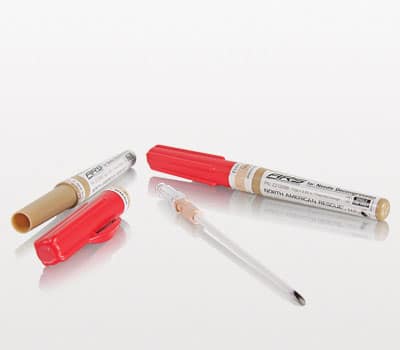
ARS for Needle Decompression (10 gauge x 3.25 in.)
$16.99
Description
- Strong, reliable 10 gauge x 3.25 in. needle/catheter for the treatment of a tension pneumothorax
- Approximately 60% larger in diameter for those seeking a larger gauge needle/catheter

- Rugged protective tube with easy-to-open textured twist top with clip
- Brown color-coded sterilization band and the catheter hub
- Capless flash chamber for immediate confirmation of needle placement
Our patented ARS® Needle Decompression Kits are designed to provide the appropriate length needle/catheter to penetrate the pleural space in 99% of patients. With its built-in failsafe characteristics, the ARS® improves the probability of success when treating casualties who present with signs and symptoms of a tension pneumothorax.
Now also available with the same quality and reliability as our current 14 gauge version, the new 10 gauge ARS® provides the option of a needle/catheter that is approximately 60% larger in diameter for those customers looking for a larger gauge needle/catheter. The brown color-coded sterilization band and the catheter hub help to distinguish it from the original 14 gauge version, which is orange.
Special Features:
- Strong, reliable needle/catheter: 10 gauge x 3.25 in.
- Rugged needle/catheter protective tube
- Brown color-coded sterilization band and the catheter hub
- Easy ID textured twist top with handy clip
- Easy-open container for quick access
- Capless flash chamber for immediate confirmation of needle placement
- Convenient, compact size
- U.S. Patents D595,847 and 001002372-0001, Patent(s) Pending
Dimensions:
- Packaged: L 5.5 in. x W 0.7 in. x 0.8 in.
- Needle Size: 10 gauge x 3.25 in.
- Weight: 0.5 oz
To watch a video on this product CLICK HERE.







Reviews
There are no reviews yet.